DEZINCIFICATION RESISTANCE OF BRASS
Dezincification is a corrosion process whereby brass, an alloy of copper and zinc, loses zinc by selective dealloying/leaching.
Dezincification can occur by either the zinc being selectively dissolved from the alloy leaving a porous copper rich structure or, depending on the environmental conditions, the copper and zinc are both dissolved with the copper then redepositing.
Dezincification results in a weakened, potentially perforated structure, but can be difficult to observe as a dezincified component may appear superficially to be intact.
Test
The Grant GD100 stirred water bath is used to provide accurate temperature control for dezincification resistance tests to BS EN 12163:2016 (formerly BS 2874:1998).
In this test, mounted and ground brass microsections are immersed for 24 hours in copper (II) chloride solution held in a beaker within a water bath at 75°C. The microsections are re-sectioned at right angles to the exposed surface, ground and polished with diamond. The polished samples are examined using an optical microscope to determine the depth of dezincification.
Specification
| Solution | 12.7g CuCl2.2H20 in 1000ml distilled water |
| Test temperature | 75±3°C |
| Test duration | 24 hours |
| Exposed test area | 100mm2 approximately |
| Test surface finish | 500 grit |
| Depth section finish | <1μm diamond |
Standards
| BS EN 12163:2016 | Copper and copper alloys. Rod for general purposes |

Dezincification of brass component leaving porous copper rich region (pink)
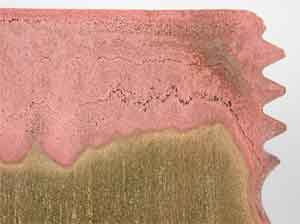
Detail of dezincified region showing potential for cracking